CHAPTER 10
RADIOLOGICAL EFFECTS
The neutron is also located in the nucleus of the
Learning Objectives: Recall the components of an
atom. It is almost as large as a proton but has no
atom; the different types of nuclear bursts and their
electrical charge.
effects; and types of personnel injuries that are caused
by blast, underwater shock, thermal radiation, and
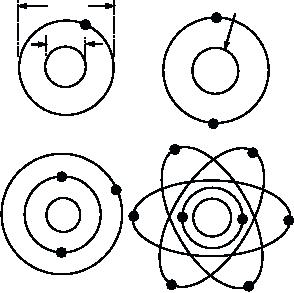
The structure of an atom resembles a solar system
with the electrons orbiting around the protons and the
nuclear radiation.
neutrons clustered tightly in the center called the
As a Damage Controlman, you will be assigned to
nucleus (fig. 10-1). Because the distance between the
a repair party during general quarters (GQ). At GQ,
electrons and the nucleus is so great, the atom is mostly
you will participate in Chemical, Biological, and
empty space. The number of electrons that orbit the
R a d i o l o g i c a l ( C B R ) c o u n t e r m e a s u r e a c t iv i t i e s
nucleus of a normal atom is equal to the number of
designed to limit the effects of a CBR attack.
protons in the nucleus. Therefore, the electrical charge
Therefore, for you to conduct your duties properly, you
of the atom is balanced. The number of neutrons in a
must possess knowledge of the basic facts about the
nucleus can vary from 0 to more than 150.
types of nuclear bursts and their radiological effects.
For more comprehensive and detailed information than
10 -8 cm
NUCLEUS
can be provided here, you should consult the Naval
E
E
10-12 m
Ships' Technical Manual (NSTM), chapter 070,
c
"Nuclear Defense at Sea and Radiological Recovery of
2P
2n
Ships After a Nuclear Weapons Explosion," and Naval
Warfare Publication (NWP) 3-20-31, "Surface Ship
E
Survivability."
HELIUM
E
E
COMPONENTS OF AN ATOM
E
Learning Objective: Recall the components of an atom.
E
3P
8n
4n
E
8P
E
E
E
Scientists have identified over one hundred
substances composed of atoms bearing an identical
E
number of protons in each nucleus that cannot be
E
E
separated into simpler substances by ordinary
LITHIUM
OXYGEN
chemical means. These substances are called
DCf1001
elements, and the smallest quantity of an element is
Figure 10-1. Rutherford-Bohr atomic models.
the atom.
An atom is made up of tiny particles known as
A process known as fission splits the nucleus of a
electrons, protons, and neutrons. The relative number
heavy element into nuclei of lighter elements. In this
of these small particles determines the attributes of an
process, an enormous amount of energy is produced.
element. The characteristics of each of these
When this energy is released in a short period of time,
subatomic particles are as follows:
an enormous explosion takes place. In the process
The electron is an extremely small particle of
known as fusion, a nuclear reaction occurs causing the
matter that orbits the nucleus of the atom. It has a
nuclei of atoms to combine (fuse together) to form a
negative electrical charge.
more massive nuclei. This reaction results in the
The proton is located in the nucleus of the atom.
release of a tremendous amount of nuclear energy. An
explosion resulting from a fission or fusion reaction is
It is approximately 2,000 times as large as an
referred to as a nuclear burst.
electron and has a positive electrical charge.
10-1

